Abstract
Background: The objective of this study was to assess current clinical practices of hematologist/oncologist (hem/onc) specialists related to chimeric antigen receptor (CAR) T-cell therapy in hematologic malignancies, in order to identify knowledge, competency, and practice gaps and barriers to optimal care.
Methods: A continuing medical education (CME)-certified clinical practice assessment consisting of 25 multiple choice questions was developed to measure knowledge, skills, attitudes, and competence of hem/onc specialists regarding CAR T-cell therapy. The survey instrument was made available online to physicians without monetary compensation or charge. Respondent confidentiality was maintained, and responses were de-identified and aggregated prior to analyses. The activity launched on December 22, 2017 with global distribution, and participant responses are still being collected at the time of abstract submission.
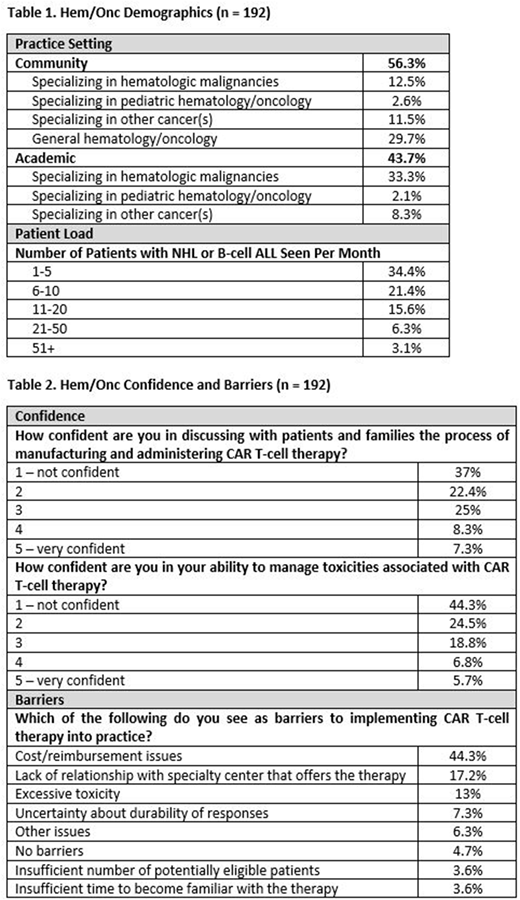
Results: At the time of this report there are 192 hem/onc activity participants, collection is on-going. Demographics are listed in Table 1 and levels of confidence and barriers to incorporating CAR T-cell therapy are listed in Table 2.
Foundational Knowledge
Sub-optimal knowledge was demonstrated in the area of CAR components, dosing, and FDA-approved indications.
Over half (61%) could not correctly identify the components of a CAR construct (antigen-specific domain and the signaling domain).
Almost half (45%) of the participants did not recognize that currently approved CAR T-cell therapies are dosed as a single infusion.
25% demonstrated inaccurate knowledge by recommending patients wait 4 weeks after CAR T-cell infusion before driving.
Over half (62%) of participants could not identify the FDA-approved indication for axicabtagene ciloleucel.
Knowledge of Clinical Trial Data
Very low awareness of efficacy data seen with various CAR T-cell products used to treat R/R B-cell ALL (ELIANA trial), R/R DLBCL (ZUMA-1, JULIET, TRANSCEND trials).
Only 32% identified the correct CR/CRi rate seen with tisagenlecleucel in the ELIANA trial.
Only 25% correctly identified the CR rate seen with axicabtagene ciloleucel in the ZUMA-1 trial.
Only 32% demonstrated knowledge of the 6-month DFS rate for patients in the JULIET trial that had a CR at 3 months.
Only 25% identified the association between the dose of JCAR017 and response rates from the TRANSCEND trial.
Knowledge and Competence Managing Adverse Events
Lack of competence recognizing and treating CAR T-cell associated adverse events such as cytokine release syndrome (CRS) and neurotoxicity.
Almost half (44%) could not identify signs of CRS associated with CAR T-cell therapy and 43% lack knowledge that elevated serum C-reactive protein (CRP) is associated with the highest level of CRS (in patients with lymphoma receiving axicabtagene ciloleucel).
41% could not identify that the mechanism of tocilizumab is to block IL-6 signaling.
Over a third (35%) were unable to identify signs/symptoms/causes of neurotoxicity associated with CAR T-cell therapy.
More than half of the learners (54%) could not identify the appropriate role of corticosteroid therapy after CAR T-cell administration in managing CRS and neurotoxicity.
Conclusions: This activity found knowledge and competence deficits for hem/onc practitioners related to using CAR T-cell therapy for the treatment of patients with hematologic malignancies. Additionally, the activity demonstrated large gaps in confidence discussing CAR T-cell therapy with patients/families and managing adverse events. There is sub-optimal awareness of CAR T-cell foundational knowledge, clinical trial data, and recognition of common therapy related adverse events and management strategies. Additional education is needed to improve the knowledge, competence, and confidence of academic and community hem/onc specialists who care for patients with hematologic malignancies receiving CAR T-cell therapy as well as strategies for integrating novel agents into clinical practice.
Neelapu:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Poseida: Research Funding; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Research Funding; Karus: Research Funding; Bristol-Myers Squibb: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Unum Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.